CLEA Japan Launches F-PDX® Mouse Preparation Service. We offer support for conclusion of MTA, generate patient-derived tumor xenograft model by engraftment of human tumor tissues to the immunodeficient mice, and propagate the models suitable for your study.
May 12, 2023, we signed a contract with the Fukushima Medical University Translational Research Center for the use of patient-derived tumor tissues (F-PDX®) to generate PDX mice.
CLEA Japan hereby starts to provide a service that covers everything from F-PDX® acquisition to generation of PDX mouse and further propagation to the experimental scale. We receive tumor tissues from the Fukushima Medical University Translational Research Center and conduct transplantation and passage in immunodeficient mice.
If you are interested in research and development of anticancer agents or cancer research with high clinical predictability using F-PDX® in vivo model, please feel free to contact us.
Service Overview
CLEA Japan supports for conclusion of MTA with F-PDX® resource center, Fukushima Medical University Translational Research Center.
CLEA Japan receives F-PDX® tissues.
Transplantation and engraftment in immunodeficient mice.
Increase to the sufficient number of animals for the test.
Deliver in the same way as for regular production animals.
Library of F-PDX®
Fukushima Translational Research Project
<F-PDX®(Patient-derived xenograft)>
1. Inquiry

Conclusion of MTA with F-PDX® Resource Hub is necessary for research use of F-PDX®, but CLEA Japan will act as an agent for purchasing F-PDX® and conducting the study on a consignment contract, so you do not need MTA with the Hub. You only have to pay to CLEA Japan in a lump sum for both acquisition of F-PDX® and preparation of mouse F-PDX® models. Additionally, CLEA Japan can also arrange a meeting between you and F-PDX® Resource representatives if necessary.
2. Preparation of F-PDX® mouse

We will prepare a sufficient number of F-PDX® mice for your study. We receive frozen F-PDX® in vials. After thawed, they are engrafted in immunodeficient mice and provided in a SPF environmental condition according to CLEA Japan's microbiological criteria. Since there is no need of propagation, you can start a study right away.
Note: It is also possible to increase F-PDX® tissue by passage in mice.
- Mice for engraftment are produced by CLEA Japan, so a sufficient number of animals are secured.
- Because the growth rate varies depending on the lineage of F-PDX®, it may take 2 to 3 months to provide.
- Through our service, you can prevent the pressures of resource, cost, and breeding space for the animal study.
Available mouse:

- Immunodeficient mouse
NOG mouse, NOD/ShiJic-scidJcl, C.B-17/Icr-scid/scidJcl, BALB/cAJcl-nu/nu
Results of F-PDX® Engraftment Confirmation Test
【Strain】 NOG mouse
【Measurement Equipment】
【Transplantation Date day0】
3. Engraftment data in CLEA Japan
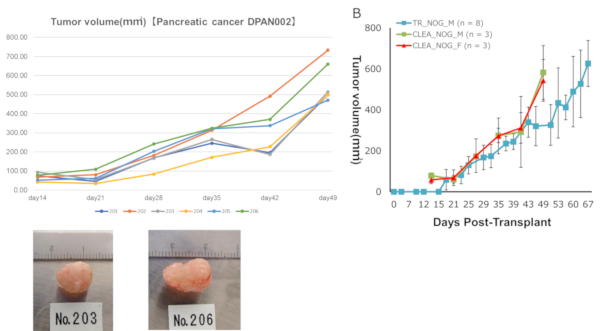
Pancreatic cancer DPAN001
Body weight
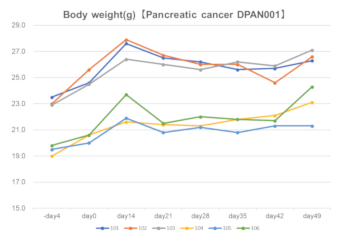
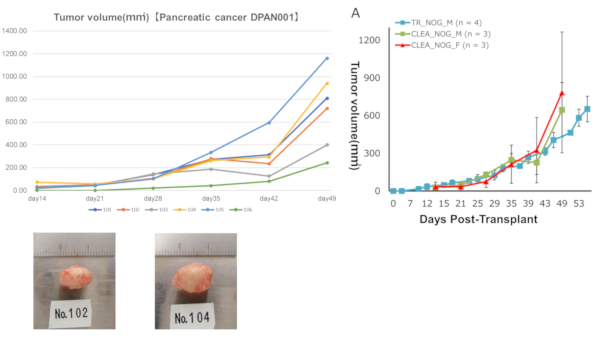
Tumor volume DPAN001
DPAN001Right: Engraftment data in Fukushima Medical University TR Center.
Lung cancer DLUN002
Body weight
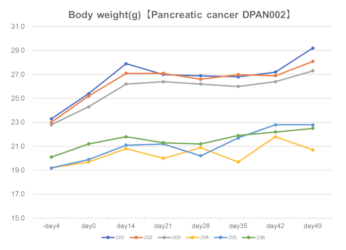
Tumor volume DLUN002
Right: Engraftment data in Fukushima Medical University TR Center.
4. Delivery of F-PDX® mouse

We will deliver the mice after the specified number of days from the transplantation date. If you need to propagation by 3 or more passages, we can present a tumor growth curve based on data obtained from the 2nd passage. Tumor growth curve would be helpful for estimating a delivery date and planning your study design.
FAQ
Is every F-PDX® capable of being engrafted in NOG mouse?If 30 animals with a certain tumor volume are required, how long does it take to deliver them?
I have not as yet had the opportunity for conclusion of MTA. How much support can you provide?
Inquiry
Note: F-PDX® is a registered trademark of Fukushima Medical University, a public university corporation.



