Research Objective
This study aimed to systematically evaluate the development of obesity and associated organ damage in C57BL/6JJcl mice following long-term feeding of our specialized high-fat diet, High Fat Diet 32 (HFD32).
*C57BL/6J mice cannot be shipped outside of Japan due to licensing agreements. Please consider using our contract research services if you need to use this strain.
Achieving highly reproducible data is critical, especially when conducting resource-intensive studies like metabolic research. We share your commitment to advancing science and understand the necessity of selecting reliable, standardized preclinical models. This study confirms the validity and strong induction rate of our C57BL/6J DIO model when fed the HFD32 diet, providing the foundational data you need to proceed with confidence and maximize the efficiency of your research.
This study aimed to systematically evaluate the development of obesity and associated organ damage in C57BL/6JJcl mice following long-term feeding of our specialized high-fat diet, High Fat Diet 32 (HFD32).
At 24 weeks of age, biopsy was performed under isoflurane anesthesia, and major organs and blood samples were collected. Blood was analyzed using an automated biochemistry analyzer (Model 3500) for the following parameters:
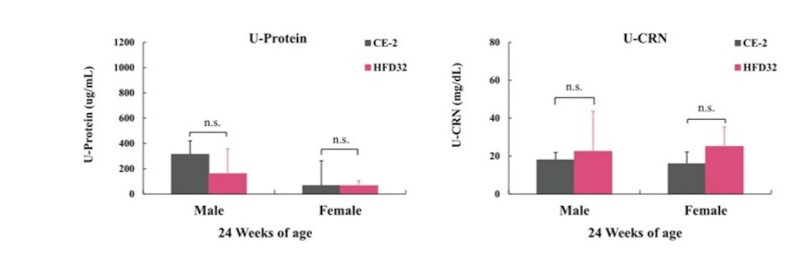
Additionally, Insulin and Glucagon levels were quantified using an ELISA kit. Urine collected via bladder puncture was measured for Urinary Protein and Urinary Creatinine. Statistical analysis was conducted using Student’s t-test, with significance levels set at P<0.05 and P<0.01.
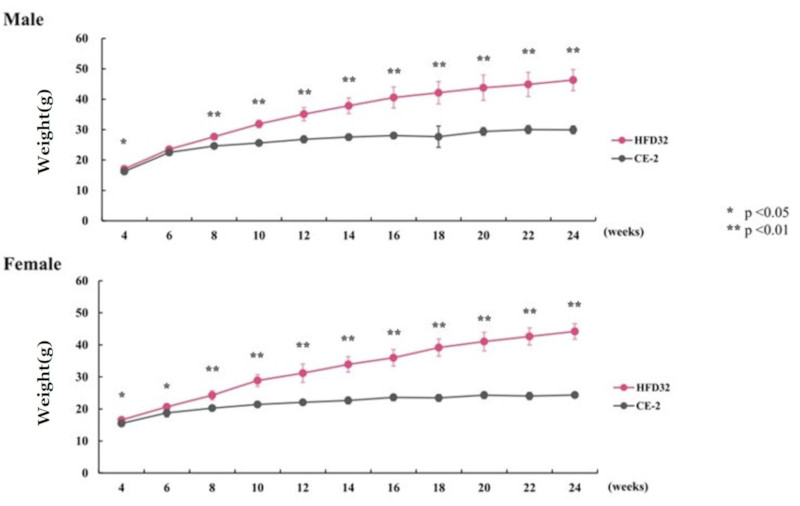
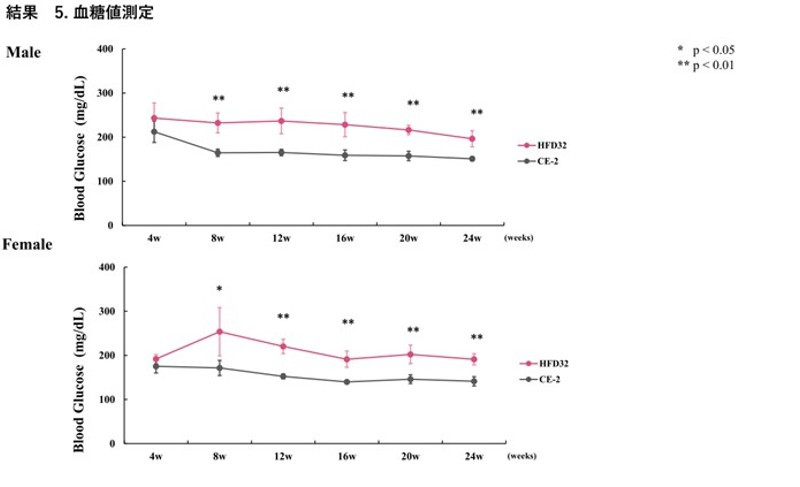
Measured bi-weekly. Points and vertical lines in the figure represent the mean ± standard deviation. Statistical calculations were performed between the test groups at each week of age (*p<0.05, **p<0.01).
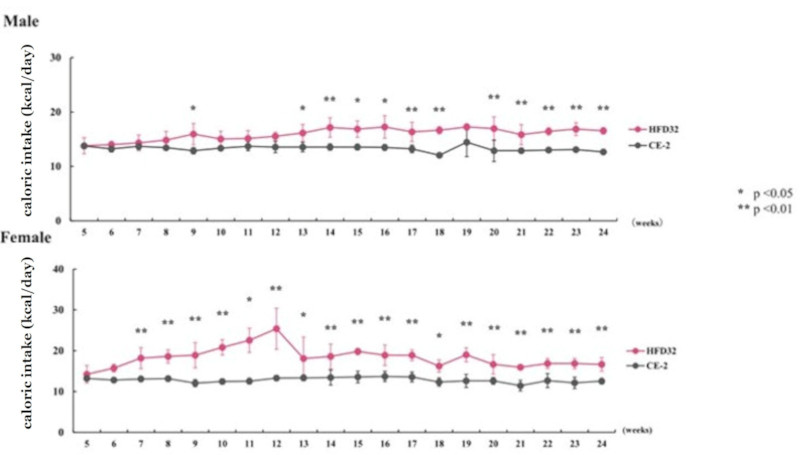
Feed intake was measured weekly, and daily caloric intake per animal was calculated. Points and vertical lines in the figure represent the mean ± standard deviation. Statistical calculations were performed between the test groups at each week of age (*p<0.05, **p<0.01).
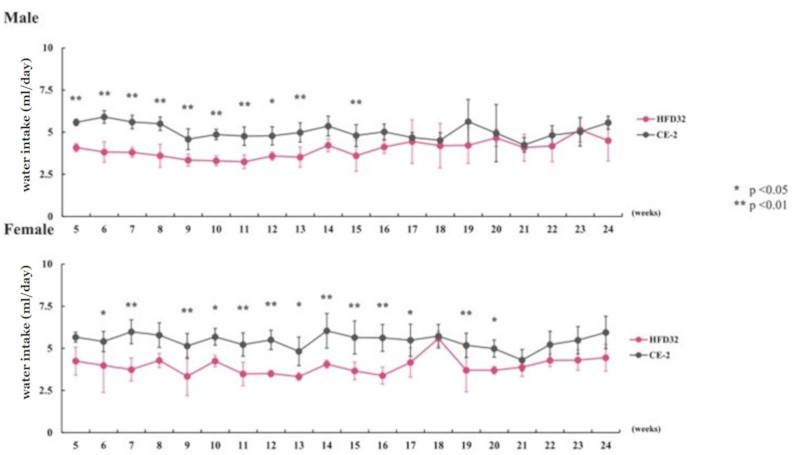
Water intake was measured weekly, and daily water intake per animal was calculated. Points and vertical lines in the figure represent the mean ± standard deviation. Statistical calculations were performed between the test groups at each week of age (*p<0.05, **p<0.01).
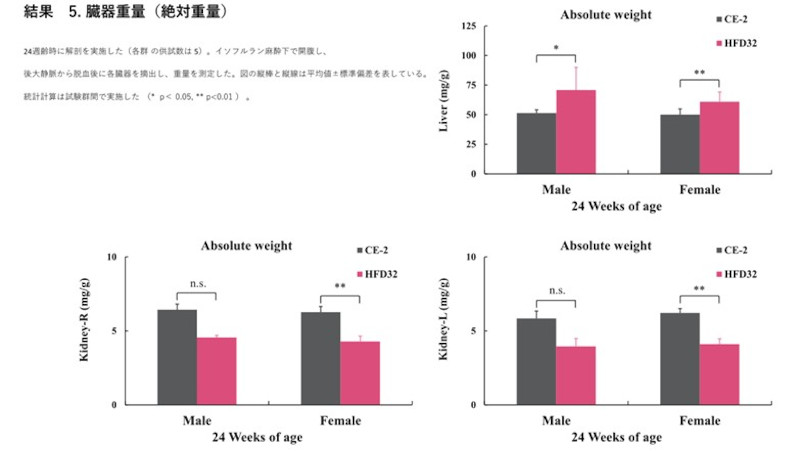
Biopsy was performed at 24 weeks of age (N=5 for each group). After exsanguination from the posterior vena cava under isoflurane anesthesia, organs were excised and weighed. Vertical bars and lines in the figure represent the mean ± standard deviation. Statistical calculations were performed between the test groups (*p<0.05, **p<0.01).
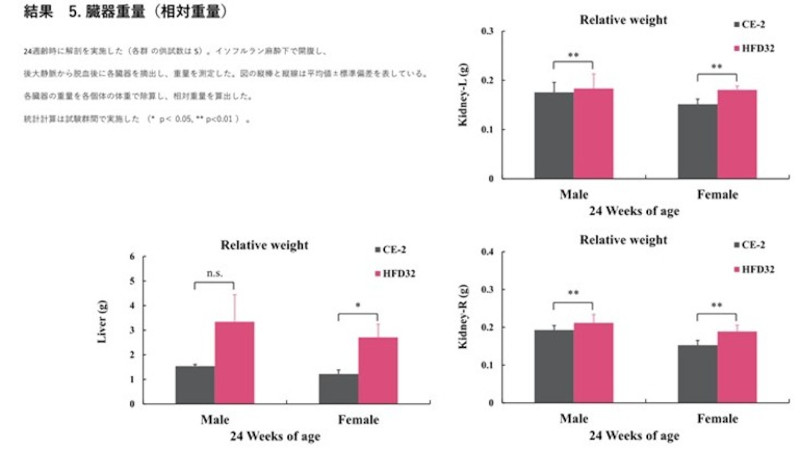
Biopsy was performed at 24 weeks of age (N=5 for each group). Organs were excised and weighed. Relative weight was calculated by dividing the absolute organ weight by the individual body weight. Vertical bars and lines in the figure represent the mean ± standard deviation. Statistical calculations were performed between the test groups (*p<0.05, **p<0.01).·
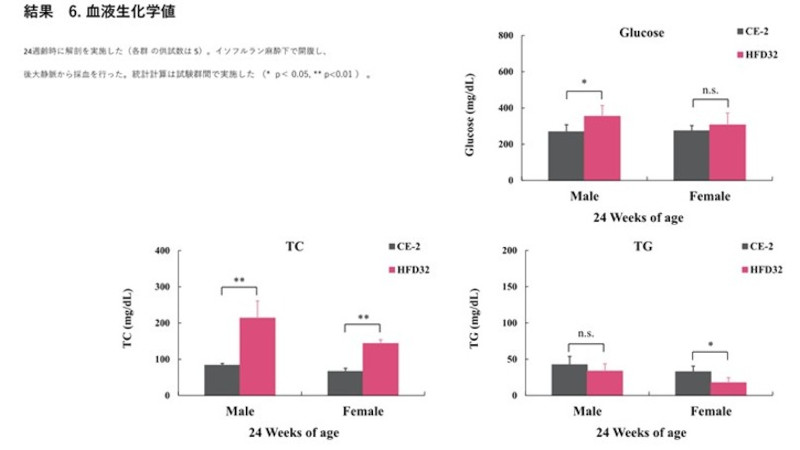
Biopsy was performed at 24 weeks of age (N=5 for each group). Blood was collected from the posterior vena cava under isoflurane anesthesia. Statistical calculations were performed between the test groups (*p<0.05, **p<0.01).
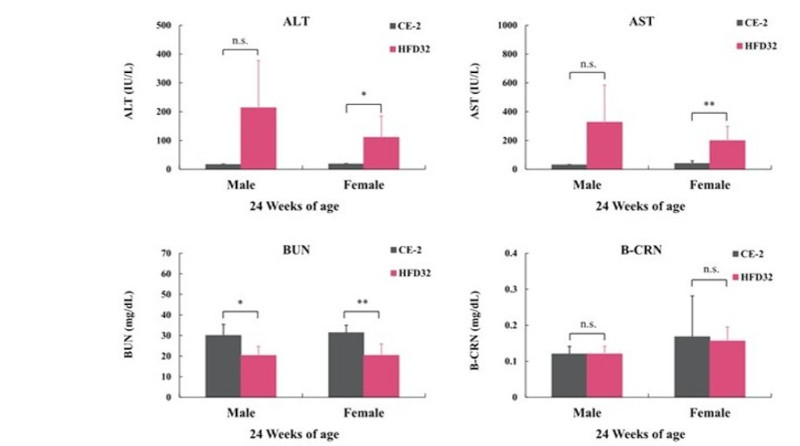
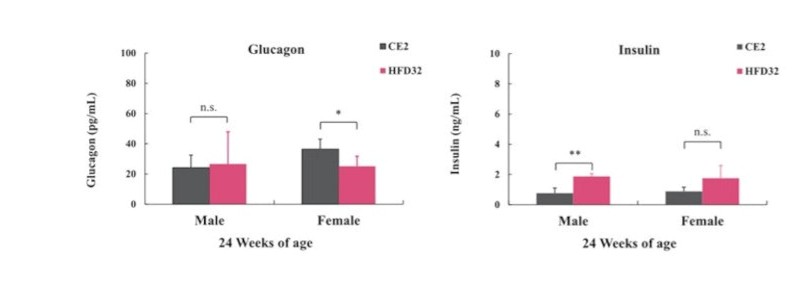
The C57BL/6J mouse clearly demonstrated high susceptibility to Diet-Induced Obesity (DIO). HFD32 feeding successfully induced obesity, glucose intolerance, and insulin resistance. Notably, when HFD32 was administered from a young age (6 weeks old), clear manifestations of obesity and metabolic abnormalities were consistently observed from approximately 8 weeks up to 24 weeks of age.
Furthermore, the significant elevated values observed in liver and lipid parameters strongly suggest the development of liver damage, such as fatty liver disease (a component of MASH/NAFLD). This protocol offers a highly reliable and reproducible system for MASH, CKD, and DKD modeling.
We want to support you to leverage these data points to streamline your experimental design and reduce unnecessary exploratory costs. Understanding the precise pathological progression in our HFD32-fed C57BL/6J model allows you to accurately time your therapeutic interventions. CLEA Japan want to be dedicated to being the reliable source for the high-quality animals and specialized diets that would ensure the success and global recognition of your findings.
Please feel free to reach out to us using the contact information below. A sales representative will be in touch shortly.
実験動物や関連の飼料、器材、受託業務に関するご質問やご相談を承ります。どうぞ、お気軽にお問い合わせください。