Introduction
CLEA Japan offers rasH2 mice, which enable short-term carcinogenicity testing with high reproducibility and reliability.
Carcinogenicity testing possible in only 26 weeks. rasH2 mice show excellent reproducibility
Carcinogenicity characteristics of rasH2 mice
- Carcinogens can be confirmed by 26 weeks of administration and subsequent pathological examinations
rasH2 mice transfected with the prototype oncogene "c-Ha-ras" of human origin show low spontaneous tumorigenicity by 34 weeks of age. It is possible to evaluate carcinogenicity by starting administration of the test substance at. 8 weeks of age and assessing tumorigenesis at 26 weeks
Since KK-Ay mice sometimes do not show increases in blood glucose levels when reared in groups, rear them for at least one week individually. When they have to be reared in groups due to spatial limitations, use them in experiments after individual acclimatization for several days.
- Carcinogen sensitivity of rasH2 mice remains stable across generations and reproducibility is excellent
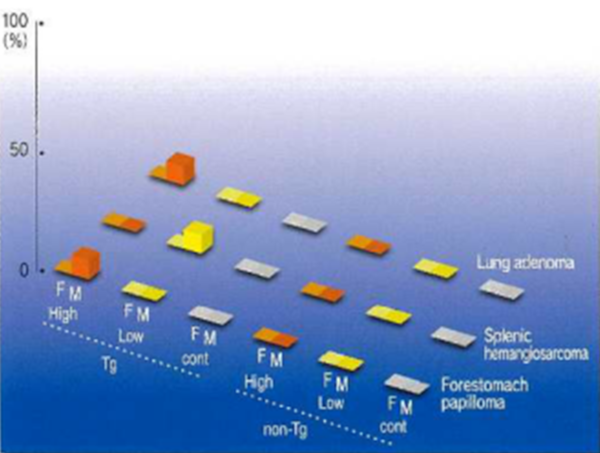
Figs. 1,2 Lind 3 show graphically tumor-susceptible organs, tumors formed and tumor incidence (%) at 26 weeks after administration of genotoxic carcinogens, non-genotoxic carcinogens, and non-carcinogens, respectively. The three columns on the left show Tg mice and the three columns on the right non-Tg mice. From the left, differences can be seen with respect to high or low doses, untreated controls or gender. In Figs. 1 and 2, tumors show a significant increase in the Tg high dose group and Fig. 3 shows no tumorigenesis with non-carcinogens even at high doses. rasH2 mice show excellent carcinogenic specificity because littermates are used as the non-Tg controls.
- Wide-ranging carcinogen sensitivity
The genetic background of rasH2 mice is an F1 hybrid of BALB/cByJ and C57BL/6J, which shows wide-ranging sensitivity to an unspecified large number of substances. The tumors formed are not. limited to lymphomas but also include epithelial and non-epithelial tumors.
Three copies of the human prototype c-Ha-ras gene with endogenous promoter/enhancer containing no artificial promoters are introduced in tandem. Stable expression of the gene has been confirmed in all major organs. The results of a complete structural analysis have shown that no instability of the gene occurs.
- Stable production system to meet all requirements
With our production system, high uniformity of the transgene, background genes and individual animals can be maintained. This system has been established to produce rasH2 mice based on high level technical achievements in the fields of reproductive and developmental engineering.
rasH2 mouse tumorigenesis patterns
Fig.1 Administration of genotoxic carcinogen-typical example
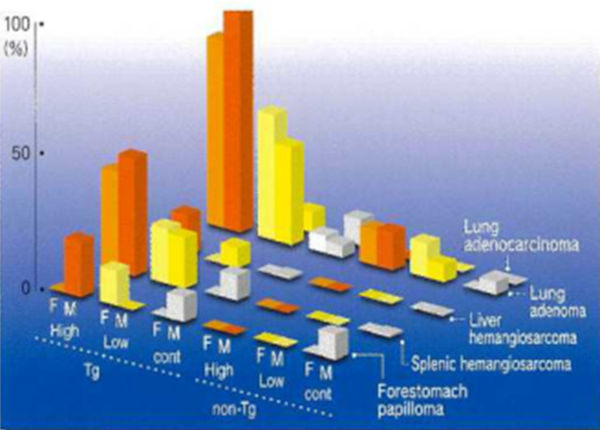
Fig .2 Administration of non-genotoxic carcinogen-typical example
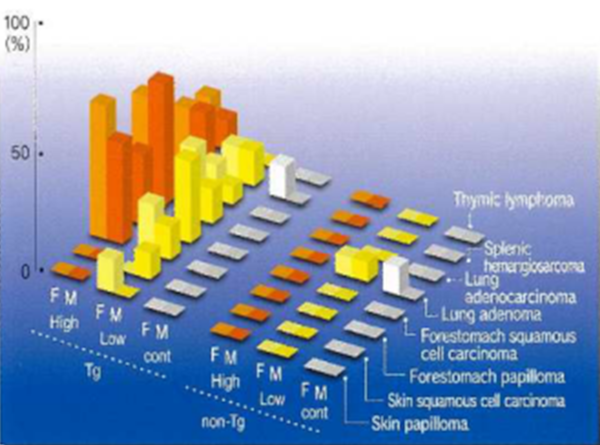
Fig.3 Administration of non-carcinogen-typical example
Quality testing of rasH2 mice
- High uniformity of background genes
Backcrossing in the foundation colony has reached more than 20 generations and the animals are still very uniform genetically. Background genes are tested regularly using C57BL/6J and BALB/eByJ genetic markers.
- High precision transgene testing
Scheduled monitoring of rearrangement (Southern blot), expression (Northern blot), translocation (FISH) or point mutations (PCR sequencing) of the transgene, as well as carcinogenicity (sensitivity to standard carcinogen, MNU) is performed. Individual animals are tested for the transgene by double PCI at 3 weeks of age to differentiate between Tg and non-Tg animals. When digestion products obtained by restriction enzymes that cut the transgene at one site are subjected to Southern blotting, bands including about 7 kb are generally obtained. When animals mated at N15 and N20 are compared, they are found to be exactly the same. Chromosomes including the transgene are examined by FISH. The transgene in both N15 and N20 are present in E3 of chromosome 15 and no translocation has been observed. Expression of the transgene shows no transgenerational changes when investigated using Northern blotting and RT-PCR.
Transgenerational Stability of Human c-Ha-ras Transgene in rasH2 mouse

Expression of the transgene
Expression of the transgene has been confirmed in all major organs.
Human c-Ha-ras gene expression in rasH2 mouse
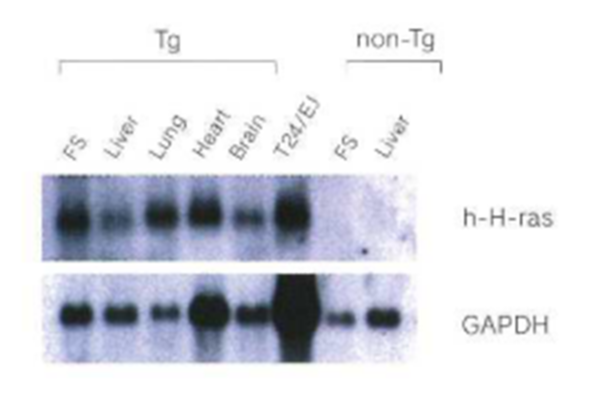
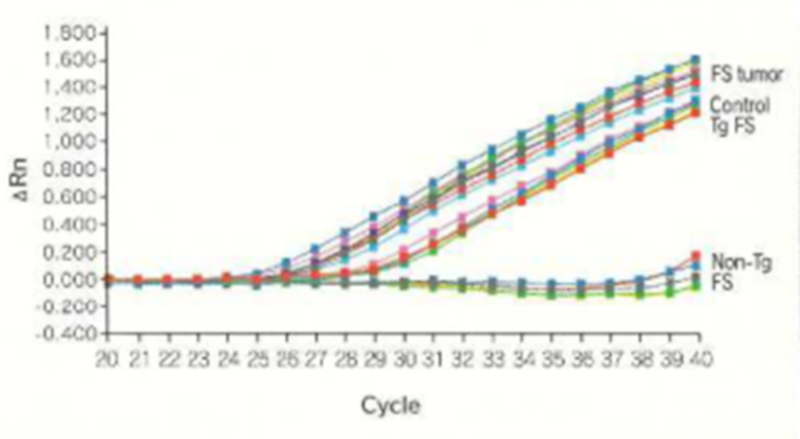
In rasH2 mice, carcinogenesis is promoted by mutations and over-expression of transfected oncogenes. The results of assays of the level of expression of the transgene iii forestomach tumors induced by ethyl nitrosourea (ENU) and methyl nitrosourea (MNIJ) using RT-PCR show an increase in c-Ha-ras.
Real-time RT-PCR for human c-Ha-ras mRNA in ENU-Induced forestomach tumor of Tg-rasH2 mice
Phenotype of rasH2 mice
- Body weight and survival rate
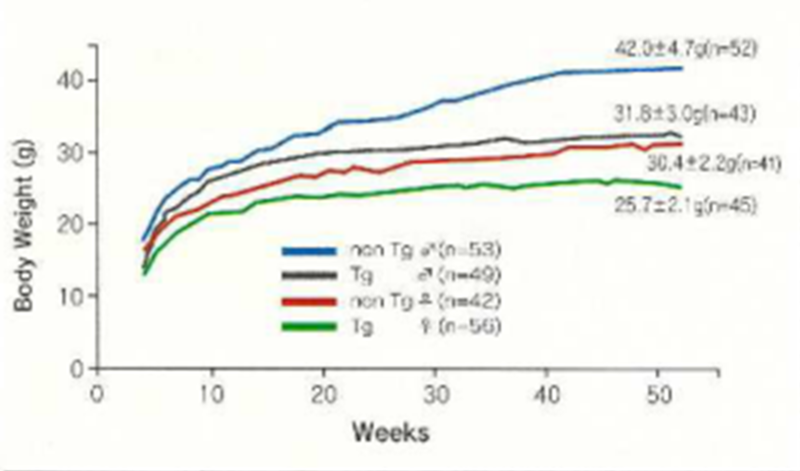
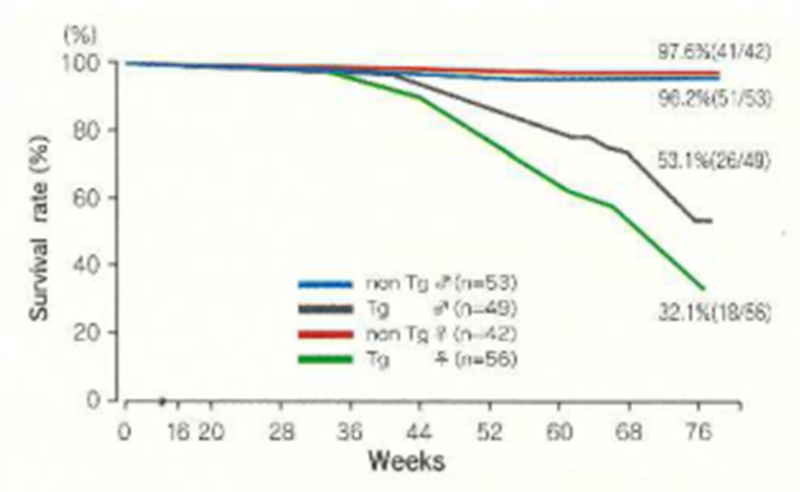
The body weights of nisi T2 mice are about 80% in males and 90% in females of those of non-Tg mice. However, the weights of each of the organs as a ratio of body weight show no differences. The survival rate is 95 to 100% at. :15 weeks and 53% for males and 32% for females at 77 weeks of age. Cancer is the cause of death in almost all animals. The homozygote of the c-Ha-ras gene is lethal.
Growth curve of CB6F1-Tg rasH2 and non-Tg mice
- Drug metabolism and hematological and biochemical profiles
No differences have been found in the hematological and bio-chemical profiles compared with the wild type. In induction of drug metabolism enzyme activity by phenobarbital or methylcholanthrene, the enzyme activity patterns of liver microsomes and cytosols show no gender differences or differences from the wild type, and no effects of gene manipulation were found.
Survival rate of CB6H-Tg rasH2 and non-Tg mice
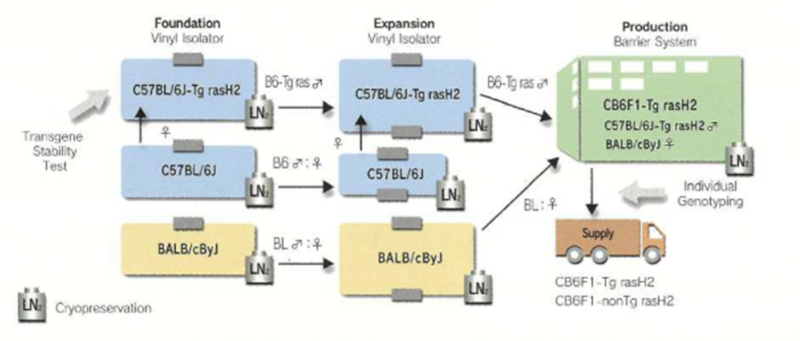
production system of rasH2 mice
The production system consists of three stages: the foundation colony, expansion colony and production colony. A backup is provided by cryopreservation of embryos.
Production system of CB6F1-Tg rasH2 mice
Background
| 1989: |
CB6F1-TgrasH2 mice created by Dr.Motoya Katsuki et al. with the support of Dr.Tatsuji Normura, director of the Central Institute for Experimental Animals (CIEA). (Part of this research was funded by the priority research project "Transgenic Animals" of the Ministry of Education, Science, Sports and Culture, Japan) |
| 1992: |
Development of a carcinogenicity testing system using CB6F1-TgrasH2 mice (rasH2 mice) started in CIEA. |
| 1996: |
Policy to replace the 2-year carcinogenicity test on mice with the short-term test decided at ICH4. |
| 1996~2000: |
High reproducibility and stability of carcinogenicity evaluations using rasH2 mice validated by international collaborative research in 50 industrial, governmental and academic facilities in Europe, Japan and the USA (ILSI/HESI) |
| 2001: |
CLEA Japan, Inc. starts production of rasH2 mice. |
Inquiry
If you have any question, please feel free to contact us from below.












