Cancer immunotherapy can be tested in the mouse immune system. Syngeneic (tumor-bearing) mice by CLEA Japan, Inc.

Cancer immunotherapy, which restores the body's natural immune system to fight cancer, is becoming a new trend in cancer treatment in recent years. In partnership with the Medical Cell Resource Center at the Institute for Gerontology, Tohoku University, CLEA Japan will produce and sell syngeneic (tumor-bearing) mouse models in Japan that have the same mouse immune function as humans. These models will be useful for studying the mechanism and basic research of cancer immunotherapy.
Characteristics

CLEA Japan’s Syngeneic mouse has significant characteristics compared to other Syngeneic mouse strains, which can be described as "2-possibilities" and "3-unnecessaries".
2-possibilities:
The animals can be purchased in the same way as usual sales animal strains. CLEA Japan will handle all the troublesome contract procedures, microbiological control, and surgical procedures. The animals can be provided quickly, flexibly, and at a low cost.
The animals will be produced at the CLEA Japan Fujinomiya Technical Service Center, which is adjacent to the animal breeding facilities. The animal model is produced quickly and flexibly.

3-unnecessaries:
- No need to acquire mouse tumor strains.
CLEA Japan will handle the MTA contract for obtaining tumor strains in the lineup below. - No need for microbiological cleaning of tumor.
All Syngeneic tumor-bearing mice delivered by CLEA Japan meet our SPF standards that can be introduced into almost any animal facilities in the world. - No need for producing tumor-bearing mice.
We will transplant your desired mouse cancer cells. No additional steps are required for mouse model creation.
The lineup of CLEA Japan's Syngeneic tumor-bearing mice.

CLEA Japan is currently capable of producing and providing the following Syngeneic mice:
| TKG No. | Cell name | Cell type |
|---|---|---|
| 0168 | P815 | Mastocytoma |
| 0144 | B16 | Melanoma |
| 0347 | B16F1 | Melanoma |
| 0348 | B16F10 | Melanoma |
| 0150 | EL4 | Leukemia |
| 0154 | L1210 | Leukemia |
| 0326 | P388 | Leukemia |
| 0158 | Meth-A | Fibrosarcoma |
| 0173 | S180 | Sarcoma |
| 0147 | Ehrlich | Breast cancer |
| 0151 | FM3A | Breast cancer |
*There is a possibility of preparing other tumor strains as well. Please feel free to inquire.
*For detailed information on tumor strains, please refer to the website of the Tohoku University Institute of Development, Aging and Cancer (IDAC) Cell Resource Center for the Biomedical Research・Cell Bank, Tohoku University.
▼ Download of Syngeneic (tumor-bearing) Mouse Model Data
Inquiries regarding delivery status, quotation requests, and other related questions.

▼ Please feel free to contact us using the form below.
* Please note that the delivery time may vary depending on the availability of tumor strains and animal species.
* Our pricing system is subject to variations based on the quantity of order.
Production of monoclonal antibodies.
The importance of antibodies in cancer therapy research is increasingly significant.
In addition to our Syngeneic tumor-bearing mouse models, we also offer you the production of mouse antibodies for mechanistic and basic research. Leveraging our expertise in animal handling and cell culture techniques developed for many years, we can produce monoclonal antibodies that meet your satisfaction within a short period (success-based fee structure).
We are also able to accommodate the production of small quantities or a wide variety of antibodies. Please feel free to contact us for inquiries.
▶ The page for our monoclonal antibody production service can be found here.
Background of this service

Since the approval of anti-PD-1 antibodies, immunotherapy that utilizes the immune system to attack cancer cells has gained attention as the fourth line of treatment following surgery, chemotherapy, and radiation therapy. Active research is being conducted in this field.
In response to this demand, CLEA Japan has formed a business partnership with the Biomedical Research Resource Center at the Tohoku University Institute of Development, Aging and Cancer.
We manage several tumor strains that have undergone microbiological testing and provide services from handling MTA administrative procedures to producing Syngeneic tumor-bearing mice on behalf of customers.
< Sales structure >
Click on the image to see more.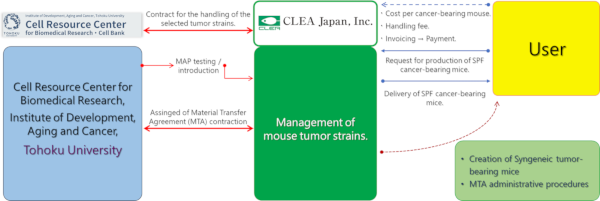
FAQ
Can you explain the procedure from ordering to delivery?
・Make and agree with the animal experiment plan
・Procure transplantable animals
・Cell culture (the duration may vary depending on the cell type and the number of animals for transplantation)
・Conclude Material Transfer Agreement (MTA) between the purchaser and Tohoku University
The above tasks usually take approximately two weeks to complete. Once completed, the tumor strains will be transplanted.
CLEA Japan is entrusted the tumor strains management by the Biomedical Research Resource Center at the Tohoku University Institute of Development, Aging and Cancer, transplantation can be initiated promptly.
Where can the cells be implanted?
For our Syngeneic models above, the transplantation is primarily performed subcutaneously, intravenously, or intraperitoneally, depending on the characteristics of the tumor. In some cases, transplantation at other parts may also be possible. Please tell us the further details.
Regarding the cell strains stored by CLEA, they have already undergone engraftment test at the instructed sites from Tohoku University. If you wish to transplant the cells to a site where engraftment test has not been conducted, we recommend performing the engraftment test before proceeding with the actual experiment.
Can you provide information about the microbiological level of the Syngeneic models?
We provide Syngeneic models that comply with our SPF standards.
Is it possible to use our own tumor strains?
If the tumor strains pass our microbiological test, we can proceed with the production of Syngeneic tumor-bearing mice.
Is it possible to create Syngeneic tumor-bearing mice using human-derived tumor cell lines or PDX tumors?
It is possible to proceed with the creation of Syngeneic tumor-bearing mice if the tumor cell lines or PDX tumors pass microbiological test and obtain approval from a human ethics committee.
Additionally, we may also be able to handle services beyond Syngeneic tumor-bearing mouse creation, such as conducting anti-tumor efficacy tests. Please feel free to consult with us through the inquiry form.
Inquiry
If you have any question, please feel free to contact us from below.


