
Introduction
CLEA Japan offers SKG/Jcl mouse, which spontaneously develop autoimmune arthritis that is immunopathologically similar to human rheumatoid arthritis (RA).
Background and Origin 1)
In 1994 a mouse with visible joint swelling was discovered within a breeding colony of BALB/c mice at Sakaguchi Laboratory. This joint swelling was judged to be caused by a genetic mutation, and Sakaguchi and colleagues began line breeding by sibling matings. Their work resulted in the SKG mouse, a genetic mutation that spontaneously develops autoimmune arthritis resembling human rheumatoid arthritis (RA). In the presence of this genetic mutation, environmental factors contribute to the onset of joint inflammation in the SKG mouse. This mouse provides a useful animal model for the analysis of etiological factors and mechanism of onset of human rheumatoid arthritis. In 1999, CLEA Japan obtained a breeding colony of SKG mice from the Institute for Frontier Medical Sciences, Kyoto University (F12 generation). The SKG/Jcl mouse was made commercially available in 2006 (F21 generation as of December 2005).
The pathology of arthritis 1)
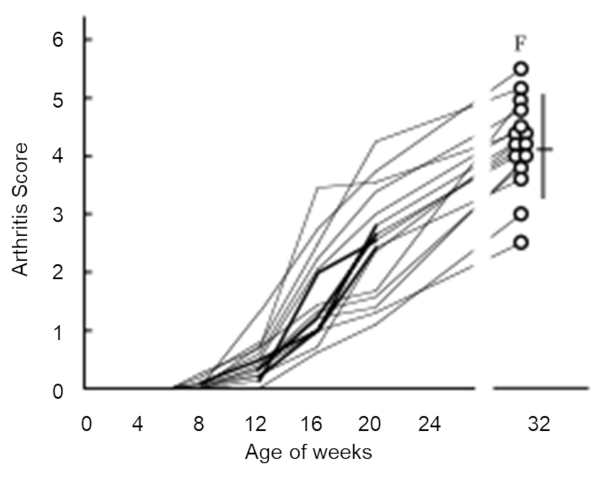
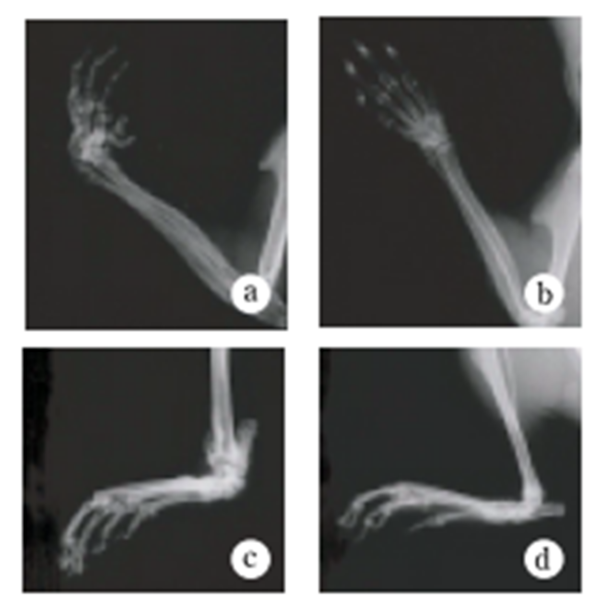
Under ordinary non-SPF conditions, the SKG mouse develops visible joint swelling from about 2 months of age, initially in the interphalangeal joints of both forepaws, and then progressing to swelling of other finger joints in the forepaws and hindpaws and to larger joints (wrists and ankles) (Fig. 1). Instances have also been observed in which the swelling began initially in the wrists and ankles, or developed simultaneously in the finger joints and in the wrists and/or ankles. Chronic progression of joint swelling is seen in both males and females, although in females the condition progresses somewhat more rapidly and tends to be more severe (Fig. 2). In an SPF environment, chronic arthritis can be induced in all animals within the same time period through antigen-nonspecific activation of innate immunity (see below). The histopathology of joint swelling in the SKG mouse is characterized initially by proliferation of the synovial membrane and massive subsynovial infiltration of inflammatory cells, followed by periarticular inflammation and formation of pannus that invades and erodes the adjacent cartilage and subchondral bone (Fig. 3). By 6 months of age most SKG mice have developed joint rigidity, and radiographic examination of the bones and joints shows loss of cartilage with destruction and fusion of the subchondral bones, joint dislocation, and osteoporosis (Fig. 4). Extra-articular manifestations of this disease in the SKG mouse include interstitial pneumonitis, dermatitis, vasculitis, and formation of a rheumatoid nodule-like lesion. (Fig. 5).
Immunological abnormalities 1)
Serologically, SKG mice develop high titers of IgM-type rheumatoid factor (RF), autoantibodies specific to type II collagen, and antibodies reactive to heat shock protein (HSP)-70 of Mycobacterium tuberculosis.
Fig.1 Macroscopic findings of arthritis in the SKG mouse
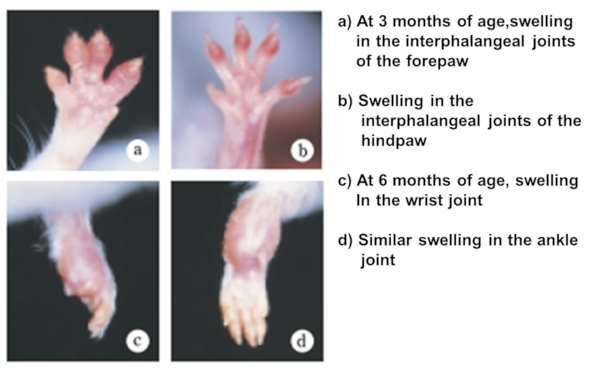
Fig.2 Progressive changes in joint swelling
Genotype and causative gene 1)
Joint inflammation in the SKG mouse is an autosomal recessive genetic abnormality that developed initially as a spontaneous mutation of the ZAP-70 gene, a key signal transduction molecule in T cells. The ZAP-70 gene has two Src homology 2 (SH2) domains, and this mutation is located in the one closer to the C terminal (the CSH-2 domain) (Fig.6).
Fig.3 Historogy of joint swelling
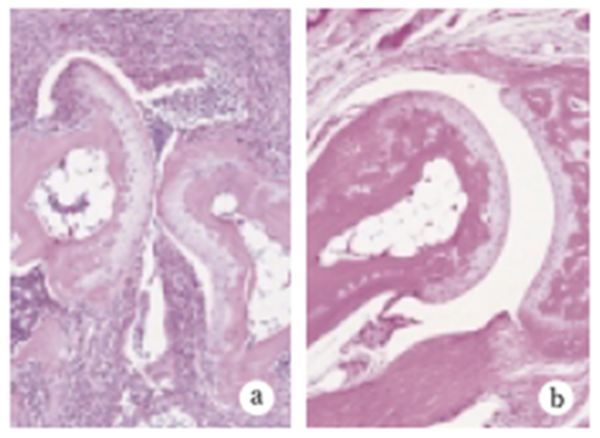
Fig.4 Joint radiography
Environmental factors in relation to joint swelling in SKG mice 3)
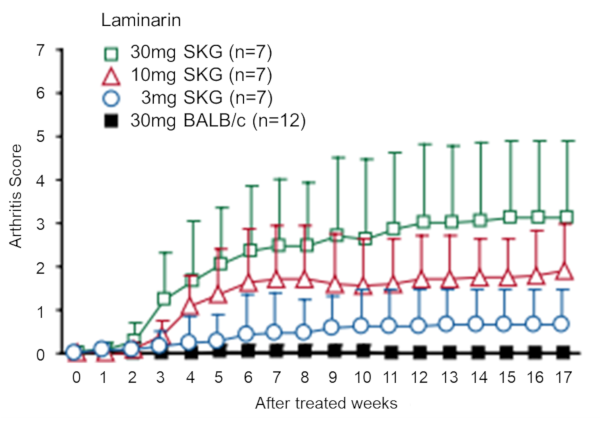
Arthritis develops spontaneously in SKG mice in an ordinary (non-SPF) laboratory environment, but is inhibited under SPF conditions. However, the thymus glands of healthy SKG mice in a SPF environment still produce T cells that have the capability to elicit arthritis, and those cells are present in the peripheral lymph tissue. In addition, chronic arthritis can be induced in all SKG mice by a single intraperitoneal administration of a β-glucan such as laminarin, even in an SPF environment (Fig. 7).
The SKG mouse thus provides a model that is useful for the analysis of genetic and environmental factors related to rheumatoid arthritis, as well as for analyzing the mechanism of onset of arthritic pathology and developing methods for the prevention and cure of rheumatoid arthritis.
Fig.5 Extra-articular lesions
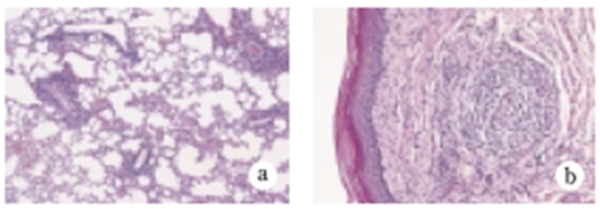
Fig.6 Causative genetic anomaly
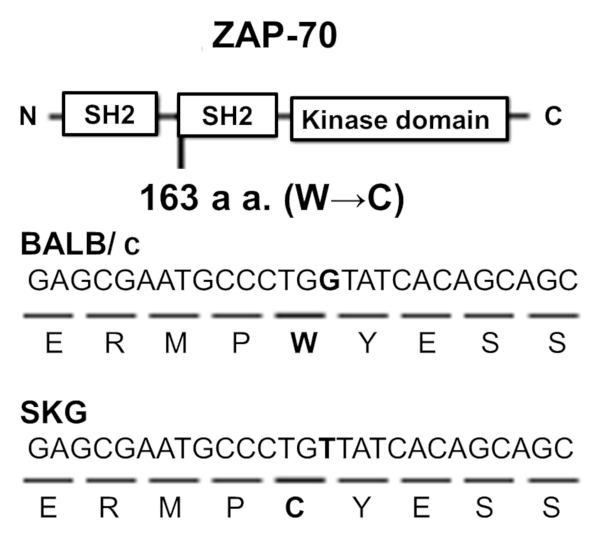
Fig.7 Elicitation of chronic arthritis by administration of laminarin
References
1) Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003; 426: 454-460.
https://pubmed.ncbi.nlm.nih.gov/14647385/
2) Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa K, Azuma Y, Kanai C, Moriizumi E, Nomura T, Nakamura T, Sakaguchi S. Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J. Clin. Invest. 2004; 114: 582-588.
https://pubmed.ncbi.nlm.nih.gov/15314695/
3) Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, Gordon S, Akira S, Nakamura T, Sakaguchi S. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp Med. 2005; 201:949-960.
https://pubmed.ncbi.nlm.nih.gov/15781585/
Confirmation of the rheumatoid arthritis-inducing effect of mannan

Inquiry
If you have any question, please feel free to contact us from below.


