CLEA Japan offers a research service for DSS-induced colitis in mice with previous laboratory background data. If you have difficulties of resource such as facilities, equipment, and/or skilled personnel, please feel free to contact CLEA Japan.
The number of patients with ulcerative colitis in Japan has been increasing since 1960, reaching 133.2 per 100,000 people in 2014. This trend is not limited to Japan, and the number of patients is increasing worldwide. However, there is still no cure.
In order to support the development and research of ulcerative colitis treatments, CLEA Japan has collected background data on DSS-induced colitis mice in our laboratory.
It is well known that the symptoms of DSS-induced colitis models can vary depending on the source of the animals and test facility/laboratory. By handling animal breeding and researching in-house, we can provide highly reproducible test results.
We can offer this services for the following customers in particular:
- For customers who want to conduct tests using a colitis model but do not have enough source of animal facilities or technicians.
- For customers who want to start testing immediately without executing prior tryout.
- For customers who want to conduct in vivo pilot tests
▼ Inquiry here

Especially if you are in any of these situations, please feel free to contact CLEA Japan.
Our experienced personnel will conduct the tests for you!
Background data:
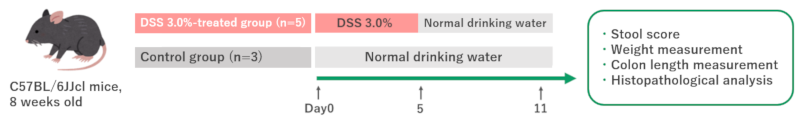
Here are the results of our background research using C57BL/6JJcl mice breeded in our facility. We compared a DSS 3.0% administration group (n=5) with a control group (n=3) of male mice at 8 weeks of age. The DSS 3.0% administration group was given DSS in drinking water for 5 days, followed by 6 days of normal drinking water. The body weights of the mice were measured and stool consistency and presence of bleeding were evaluated. On day 11, the animals were euthanized and the colon was sampled. We measured colon length, and histopathological evaluation[1].
The data were analyzed for statistical significaces using Student’s t-test. Difference was asseced with two-side test with an α level of 0.05 Values are means ± SD of independent experiments. Asterisk indicates a significant difference (p < 0.05).
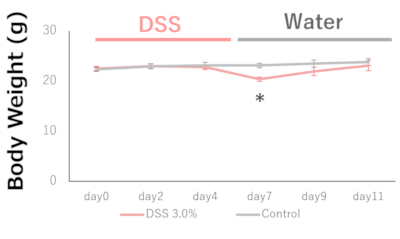
Growth curve
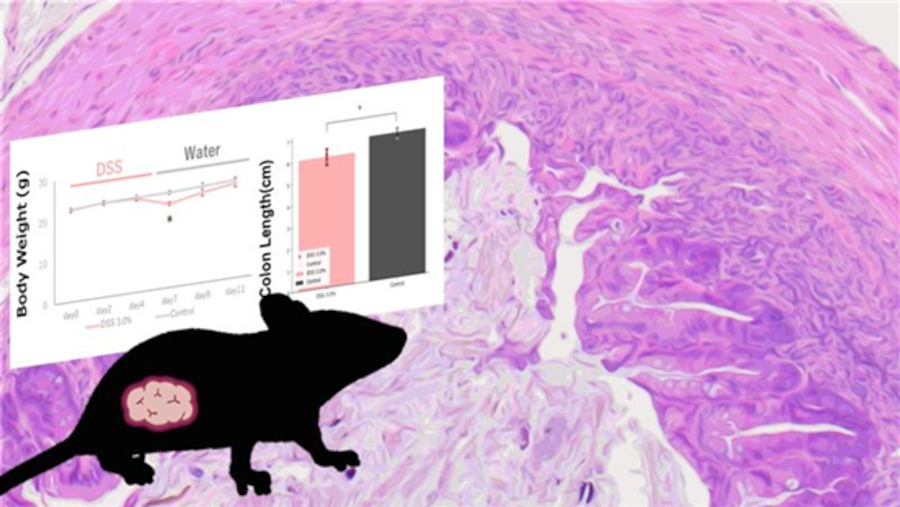
Weight loss, a symptom associated with ulcerative colitis, was confirmed in the DSS 3.0% administration group.
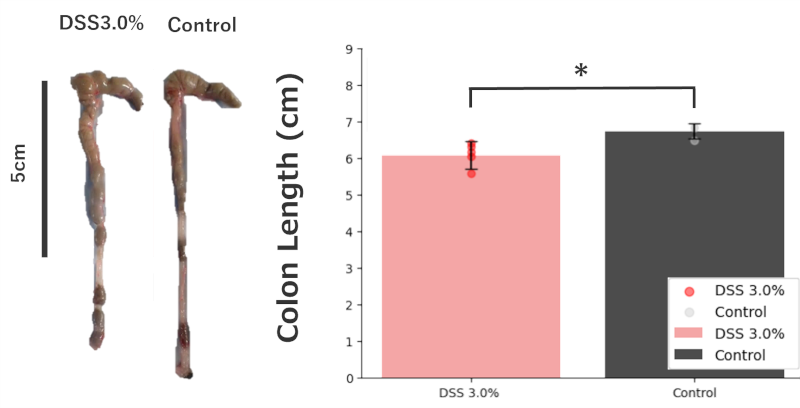
Colon Length:
The DSS 3.0% administration group showed a significant shortening of colon length compared to the control group.
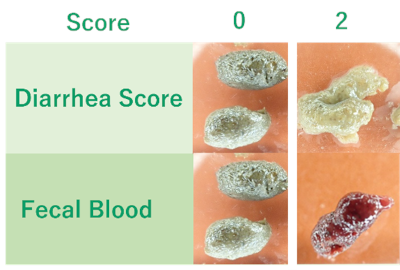
Stool Consistency, Rectal Bleeding:
Scoring was performed based on the stool consistency and presence of bleeding.
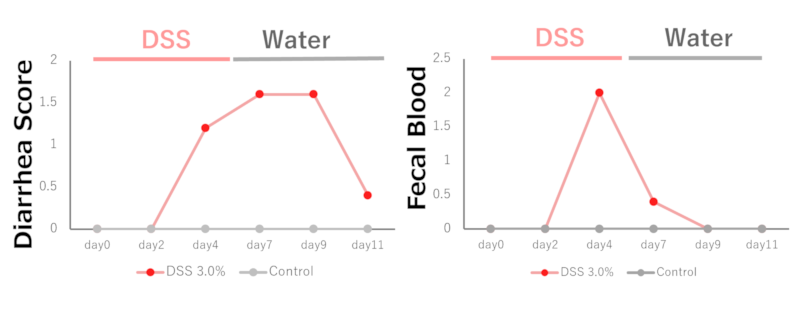
In the DSS 3.0% administration group, diarrhea and loose stool symptoms were observed on the 4th day, and the symptoms then improved.
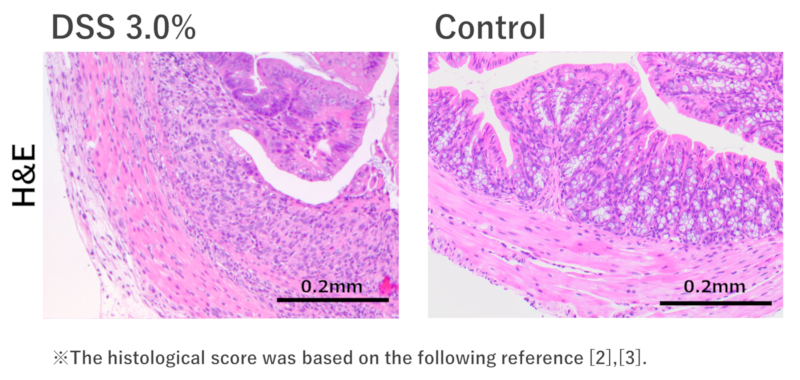
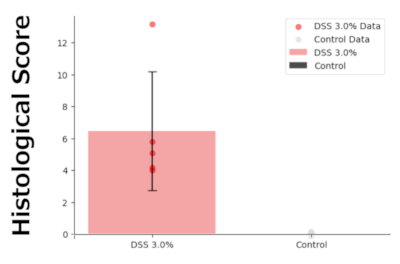
Histological analysis:
The DSS 3.0% administration group showed symptoms of colitis, such as crypt damage, erosion, muscle thickening, and infiltration of inflammatory cells. No significant changes were observed in the control group.
In the DSS 3.0% administration group, inflammation of the colon was confirmed.
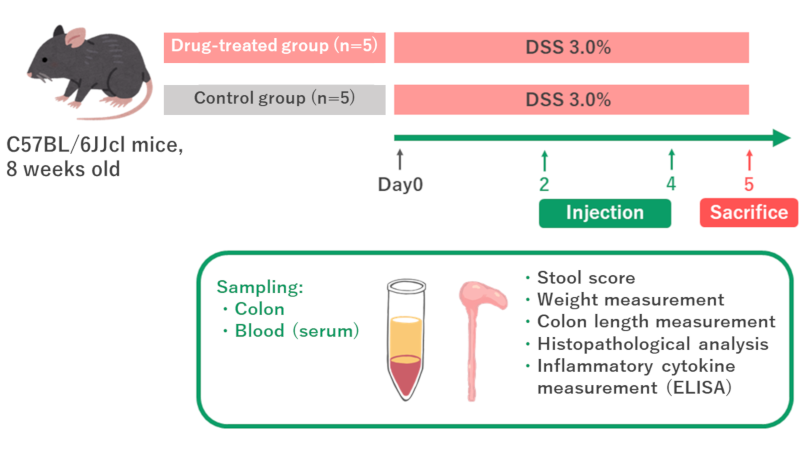
Sample of study design:
We have set up a plan for a DSS-induced colitis study.
- Drug Administration and monitoring:
Tail vein injection will be performed, and the stool and body weight are monitored every other day from the start of the test to the final sampling. - Sampling:
Colon and blood (serum) samples will be collected from the mice on the last day of the study. - Measurement and Analysis:
Colon length will be measured and histological evaluation will be performed*.
Inflammatory cytokines and other biomarkers in serum will also be measured by ELISA.
*For customers who require histopathological evaluation for this service, we will assist you in finding a company to analyze the colon samples collected and fixed by our company, and even outsource it.
Please contact us for a estimation.
References
FAQ
I conducted a test using CLEA's mice under the same conditions as the background data in my own facility, but the symptoms were heavier/lighter than the data.
Inquiry
If you have any question, please feel free to contact us from below.